Introduction
Influenza, commonly known as the flu, is an acute respiratory infection caused by influenza viruses,1 characterized by sudden onset, rapid transmission, and the potential to reach epidemic levels. Influenza-like illness (ILI) is a…

Influenza, commonly known as the flu, is an acute respiratory infection caused by influenza viruses,1 characterized by sudden onset, rapid transmission, and the potential to reach epidemic levels. Influenza-like illness (ILI) is a…
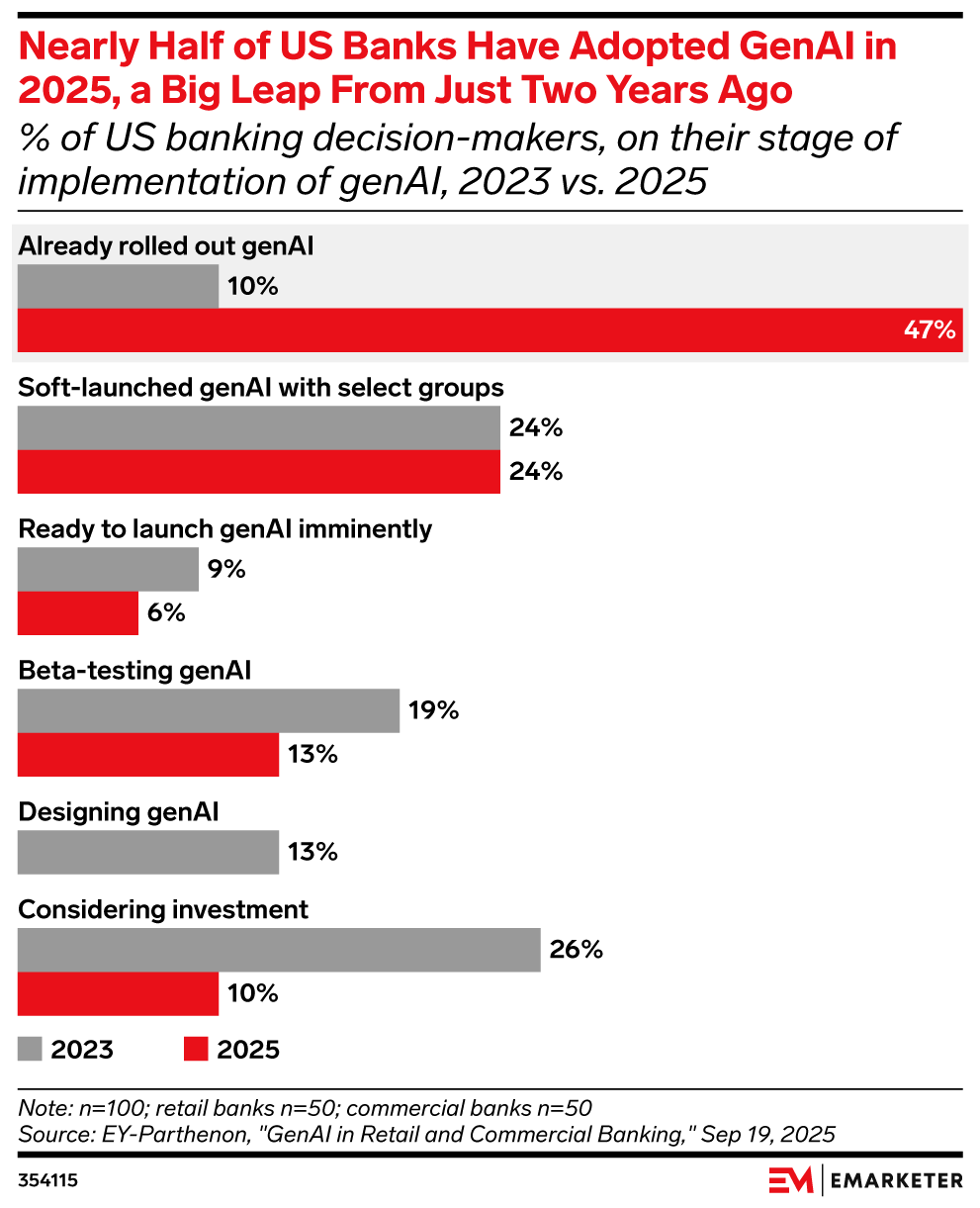
Key stat: 47% of US banking decision-makers say their institutions have already will have fully rolled out generative AI, up from 10% in 2023, said data from EY-Parthenon.
Beyond the chart:
Use this chart: This is the time to move from pilot projects to full deployment. Laggards risk falling behind in customer experience, cost savings, and innovation. Strategy teams should benchmark where they stand against peers and identify quick-win use cases (like chatbots or risk modeling) to accelerate adoption.
Related EMARKETER reports:
Methodology: Data is from the July 2025 EY-Parthenon report titled “GenAI in Retail and Commercial Banking.” 100 US banking employees were surveyed during March 2025. The sample included 50 respondents from retail banks and 50 from commercial banks, all with direct involvement in or knowledge of genAI initiatives. Respondents held roles in client servicing, marketing, onboarding, product strategy, investment, or technology. Titles included C-level executives and heads of departments tied to genAI applications such as ChatGPT, DALL-E, OpenAI, and Microsoft Azure.


A chaotic nine months at the FDA has witnessed the toppling of more than half the agency’s senior leadership, the axing of 3,500 more staff and a safety scandal that upended the gene therapy sector, which was followed by the subsequent ouster—and surprising return—of CBER Director Vinay Prasad. But the first three quarters of 2025 have seen an average number of new drug approvals, according to an analysis by Jefferies.
“Fears of a dysfunctional FDA may be overblown,” the analysts wrote to investors on Aug. 25. “We think the FDA’s actions YTD have not signaled a more stringent or irrational agency.”
The Jefferies group counted 28 total approvals as of that date between the Center for Drug Evaluation and Research (CDER) and Center for Biologics Evaluation and Research (CBER) combined and extrapolated out to 43 by the end of 2025, a number that would fit squarely into the range of 37–68 over the past 5 years.
“The bottom line is, we didn’t sense anything drastically alarming or different compared to prior years,” Jefferies analyst Andrew Tsai told BioSpace in an interview last month.
As for rejected drug applications, these were trending lower than last year, Jefferies reported Aug. 25, thanks to CDER being on pace to issue fewer CRLs compared with the past five years. CBER, on the other hand, was heading toward issuing more CRLs.
The acceleration of therapies for rare diseases to the market has been a top-stated priority of FDA Commissioner Marty Makary since he took office in April. Makary first floated the idea of a conditional approval pathway for such therapies in one of his first media interviews, and the FDA has since rolled out the Rare Disease Evidence Principles, a new framework meant to streamline the approval of drugs for ultra-rare diseases.
“They seem to be following it through with what they want to achieve, especially within the rare disease side, and expediting drugs and so forth,” Tsai said.
This year, the regulator has approved 14 novel therapies for rare diseases—including ones for alkaptonuria, recessive dystrophic epidermolysis bullosa and phenylketonuria. On the other hand, seven others received complete response letters, some of which came as quite a surprise to the company executives and other experts.
Capricor Therapeutics CEO Linda Marbán was caught off-guard after the company’s cell therapy for Duchenne muscular dystrophy was rejected in July, saying on Capricor’s second quarter earnings call the next month, “The complete response letter was unexpected given the trajectory of positive interactions [with the FDA].” And 22 scientists who designed and ran the trials of Replimune’s advanced melanoma drug, RP1, wrote an open letter urging the FDA to reconsider its July rejection.
Stealth BioTherapeutics has had success getting the FDA to reconsider its May rejection of Barth syndrome drug Forzinity. After refiling in August, the company was granted an expedited review and won accelerated approval less than a month later.
The highest number of novel drug approvals this year have come from the oncology space, according to data collected by both BioSpace and Jefferies. Treatments for infectious diseases and autoimmune/immunological diseases are also high among this year’s FDA approvals.
In the past 25 years, control of the White House has jumped back and forth from Republican to Democrat multiple times. During three of those changeovers, a new FDA commissioner was installed during the administration’s first year. As drug approvals are not political decisions, “You would not expect a flood of new drug approvals in the inaugural year of a new administration,” suggested Steven Grossman, policy and regulatory consultant and author of the FDA Matters blog.
This has proven to be the case, Grossman said, except in 2017, during President Trump’s first term. That year, when Scott Gottlieb took the reins from Robert Califf, who served as commissioner during Barack Obama’s second term, the FDA approved 46 new drugs—more than double the number greenlit the previous year.
“The last two years of an eight-year administration are not known for policymaking or starting new initiatives,” Grossman told BioSpace. “I would not expect that to have slowed drug approvals in 2015 or 2016 or led to a surge of approvals in 2017.”
Indeed, in 2009, when Margaret Hamburg took the helm at the FDA during Obama’s first year in office, the agency approved just one more novel drug than in 2008. The results of this year’s changeover—from Califf, who served a second stint as commissioner, to Makary—are still-to-be-determined.
Potentially adding a new wrinkle to future data analysis attempts is the current U.S. government shutdown. The FDA announced on Oct. 1 that it would be unable to accept any new drug applications until after the shutdown is resolved—as to do so would require the agency to accept industry user fees assessed for 2026, which it is unable to do during this “lapse period,” Leerink Partners explained in a note to investors that morning.
Only time will tell if this has any bearing on 2025’s ultimate approval tally.
New analysis from Jefferies shows that rare disease and cancer drugs granted the status are especially likely to be approved.

Lindsay points toward an important issue: the disconnect between research on the sensory processing of complex, natural visual information and…

Intel has confirmed it is developing a “Next ARC Family” of GPUs, utilizing its “Xe3P” graphics architecture. This news, revealed at the 2025 Tech Tour US, follows an in-depth look at Intel’s Xe3 GPU architecture, which is…

Image: (From left) Prof Mary Chan from Nanyang Technological University, Singapore, Dr Kaixi Zhang from the Singapore-MIT Alliance for Research and Technology, and Prof Paula Hammond from the Massachusetts Institute of Technology were…

The intervention also raised blood serotonin, the neurotransmitter 5-HTP helps produce. However, anxiety scores and Alzheimer’s-linked blood proteins did not change.
The study enrolled 30 adults with a mean age of 66 to 67. Participants were…
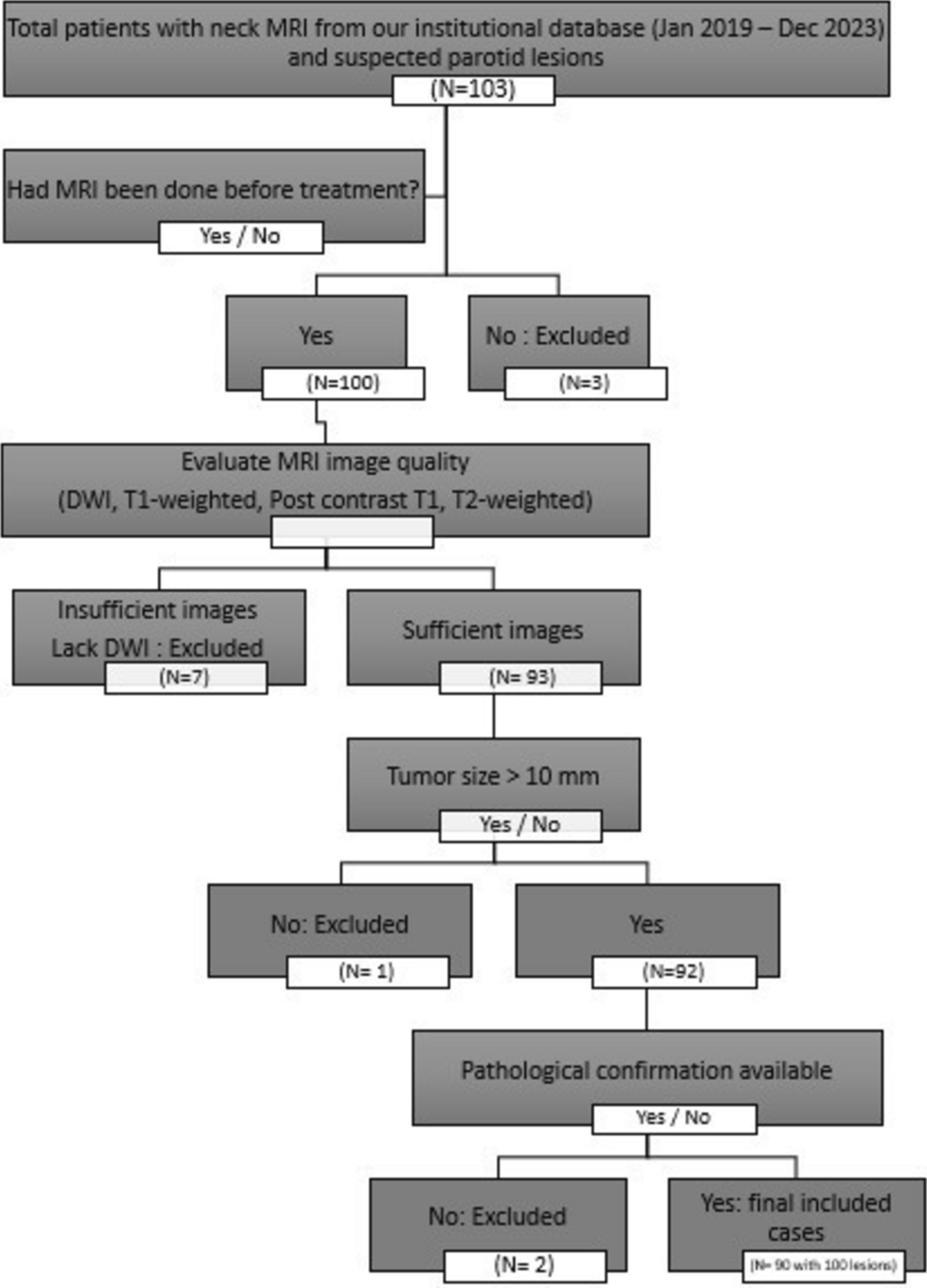
Distinguishing between benign and malignant parotid tumors remains a clinical challenge. Moreover, the true extent of tumor infiltration is often underestimated upon clinical examination [5].
This study aimed to evaluate the diagnostic performance and inter-observer reliability of conventional MRI and ADC histogram analysis in differentiating benign from malignant parotid gland masses.
In harmony with our findings, Rajbhar et al. [6] conducted a five-year retrospective analysis of 67 surgically excised salivary gland tumors to evaluate demographic, clinical, and histopathological patterns. They reported a male-to-female ratio of 1.1:1, indicating a slight male predominance consistent with broader trends in salivary gland tumors. They also found a higher proportion of benign tumors, with 85.1% benign and only 14.9% malignant cases, and the parotid gland was the most frequently affected site, accounting for 76.1% of tumors, with benign tumors predominantly arising from this gland (80.7%), supporting its established role as the primary site of salivary neoplasms.
In concordance with our findings, Tartaglione et al. [7] retrospectively evaluated pre-surgical MRI findings in 94 patients with histologically confirmed parotid gland tumors to identify imaging characteristics predictive of malignancy and found that the majority of cases were benign 69 (73%), while 25 (27%) cases were malignant, and most tumors in their cohort were unilateral. Also, they identified multifocal lesions in three Warthin’s tumors and one pleomorphic adenoma, indicating that multifocality, while less frequent, can occur in both benign and malignant pathologies.
Parallel to our results, Xu et al. [8] conducted a retrospective MRI-based study on 73 patients with histologically confirmed parotid tumors and found that for ADC value, the intraclass correlation coefficient was 0.85 and P value < 0.001, indicating an excellent agreement between the two observers.
In harmony with our findings, Takumi et al. [9] conducted a study using diffusion tensor imaging (DTI) to assess its ability to differentiate malignant from benign parotid gland tumors. They evaluated 59 tumors using ADC and fractional anisotropy (FA) metrics at 3 T MRI and reported a mean ADC value of 0.93 ± 0.21 × 10⁻3 mm2/s for malignant tumors and 1.19 ± 0.50 × 10⁻3 mm2/s for benign tumors, with no statistically significant difference between them (P = 0.225). Although the difference did not reach significance in their sample, their numerical trend toward lower ADC in malignancy supports the directionality observed in our study. They also found an inter-observer agreement which was excellent, with ICCs of 0.98 for both ADC and FA.
In addition, Zhang et al. [10] included 67 patients with 71 salivary gland tumors who underwent MRI examination to evaluate the diagnostic performances of DWI and intravoxel incoherent motion (IVIM) for discriminating between benign and malignant tumors and found that an analysis of inter-observer agreement showed that for all DWI- and IVIM-related parameters, the mean values showed the highest ICCs (0.992 [95% CI: 0.987–0.995] for ADC mean (P < 0.001).
In our study, malignant lesions were associated with older patient age and significantly lower ADC values, including mean, minimum, and maximum ADCs, along with higher skewness, indicating asymmetric diffusion.
In partial agreement with our results, Wang et al. [11] conducted a retrospective study of 526 patients to differentiate benign from malignant parotid tumors using clinical and ultrasound features alongside radiomics and deep learning models. They reported no significant difference in gender distribution between groups (male: 53.80%, female: 46.20%; P = 0.923), consistent with our findings. Similarly, calcification showed no significant association with tumor type (P = 0.264). In contrast, they found no significant difference in margin definition (P = 0.176) or cystic component presence (P = 1.0). This discrepancy may be attributed to the higher soft tissue contrast and structural detail provided by MRI compared to ultrasound.
Supporting our results, Razek et al. [12] performed a prospective study involving 27 patients—18 with pleomorphic adenomas and 9 with salivary gland malignancies, to evaluate the role of DWI and histogram analysis of ADC maps in characterizing parotid tumors. Whole-lesion ADC histogram parameters were assessed, revealing significantly higher ADCmean (1.93 ± 0.34 vs. 1.26 ± 0.54, P = 0.007), ADCmin (0.96 ± 0.29 vs. 0.62 ± 0.16, P = 0.02), and ADCmax (2.26 ± 0.36 vs. 1.52 ± 0.56, P < 0.001) in the pleomorphic adenoma group compared to the malignant group. Contrastingly, skewness was significantly greater in pleomorphic adenoma (0.57 ± 0.56 vs. 0.37 ± 0.52, P < 0.001), while no significant difference in kurtosis was observed (P = 0.86).
Also, Chen et al. [13] conducted a retrospective study to evaluate the diagnostic performance of histogram features derived from DWI, diffusion kurtosis imaging (DKI), and IVIM for differentiating benign from malignant parotid gland tumors. They reported significantly lower ADCmean and ADCmedian values in malignant tumors compared to benign ones (982.81 vs. 1194.39 × 10⁻⁶ mm2/s and 968.38 vs. 1189.38 × 10⁻⁶ mm2/s, respectively; P = 0.02 for both). They also reported no significant differences in kurtosis between malignant and benign lesions in ADC-based analysis (P = 0.18).
In another study population, Zhu et al. [14] conducted a retrospective study aiming to differentiate benign from malignant palatal lesions using conventional MRI features and ADC histogram analysis. The study included 86 histopathologically confirmed patients (57 with malignant and 29 with benign palatal lesions). It assessed nine ADC histogram parameters derived from DWI, alongside conventional MRI features such as lesion size, capsule presence, and nerve invasion. They found that malignant lesions had significantly lower ADC50 (1.01 ± 0.16 × 10⁻3 mm2/s vs. 1.51 ± 0.33, P < 0.001), ADC10 (0.79 ± 0.15 vs. 1.22 ± 0.29, P = 0.001), and overall mean ADC (1.02 ± 0.19 vs. 1.51 ± 0.37, P < 0.001) compared to benign lesions, which mirrors our observation of consistently lower ADC values across various measures in malignant tumors. Additionally, they reported significantly larger lesion size in malignant cases (P = 0.02), as well as higher frequency of nerve invasion (P = 0.022) and absent capsule (P = 0.019).
In our study, both observers demonstrated high and consistent diagnostic performance in using ADC values to differentiate malignant parotid lesions, with excellent agreement. At the optimal cut-off value of < 1.15 × 10⁻3 mm2/s, observer 1 achieved an AUC of 0.847 and observer 2 an AUC of 0.857, with closely matched sensitivity, specificity, and accuracy values. This strong alignment indicates a high level of interobserver concordance in ADC-based malignancy assessment.
In harmony with our findings, Al-Kheshen et al. [15] investigated the role of diffusion-weighted MRI and ADC values in differentiating benign from malignant salivary gland tumors. They reported a significantly lower mean ADC in malignant lesions. They achieved high diagnostic performance using a cut-off value of < 0.85 × 10⁻3 mm2/s, with a sensitivity of 93.7%, specificity of 95.8%, and overall accuracy of 94.4%. Although their metrics were higher, the discrepancy may be attributed to differences in imaging parameters, cut-off thresholds, and population characteristics. Nonetheless, both studies confirm the strong diagnostic utility of ADC in parotid tumor characterization.
In addition, Aly Nada et al. [16] investigated the role of DWI and ADC in distinguishing between benign and malignant parotid gland lesions. They reported a statistically significant difference in ADC values, setting a cut-off at 0.93 × 10⁻3 mm2/s, which yielded a sensitivity of 72.1% and specificity of 82%, closely aligning with our diagnostic performance.
Moreover, in another population, Eida et al. [17] assessed the diagnostic value of ADC mapping in distinguishing benign from malignant salivary gland tumors in a cohort of 31 patients and found that malignant tumors exhibited significantly fewer areas with high ADC values (≥ 1.8 × 10⁻3 mm2/s). Using a threshold of high ADC occupying < 5% of the tumor area, they reported a sensitivity of 89%, specificity of 100%, and an overall accuracy of 97%. While their method involved spatial analysis of ADC distribution rather than a single cut-off value, it also underscores the utility of ADC in preoperative differentiation of salivary gland lesions, with methodological differences likely explaining the higher accuracy reported in their work.
Also in our study, ROC analysis revealed that mean ADC was the most reliable histogram parameter for differentiating malignant from benign parotid lesions., with an AUC of 0.793, 75.6% sensitivity, 67.8% specificity, and 74.3% overall accuracy. Minimum ADC showed fair diagnostic ability (AUC = 0.720) with slightly lower specificity and accuracy. Maximum ADC had the highest sensitivity (80.5%) but the lowest specificity (59.3%) and overall diagnostic performance (AUC = 0.712, accuracy = 67.2%).
In agreement with our findings, Razek et al. [12] identified mean ADC as the most accurate parameter, reporting an AUC of 0.948 with 88.9% sensitivity and 94.4% specificity at a cut-off of 1.16 × 10⁻3 mm2/s. Their minimum ADC also showed comparable diagnostic performance (AUC = 0.753, sensitivity = 88.9%, specificity = 61.1%), aligning with our results. Similarly, maximum ADC was the least discriminative in both studies, with their AUC of 0.873 slightly exceeding ours, likely due to population differences and tumor subtype stratification.
In partial agreement with our findings, Hepp et al. [2] conducted a prospective study involving 73 patients to compare the diagnostic performance of full ADC histogram distribution curves against mean ADC values for parotid tumor differentiation. They reported that the mean ADC provided higher sensitivity than histogram distribution (71.4% vs. 61.9%), aligning with our finding that mean ADC was the most sensitive individual parameter. However, they found that histogram-based analysis offered superior specificity (75.0% vs. 71.2%) and higher predictive accuracy during cross-validation (71.2% vs. 67.1%). This contrast in specificity may be attributed to their use of an advanced full-curve comparison approach.
However, Sobhy et al. [18] conducted a prospective study on 25 patients using combined conventional MRI and DWI to evaluate the diagnostic value of ADC in parotid tumor characterization. They reported markedly higher diagnostic accuracy, with a sensitivity of 92.86% and specificity of 90.91%, alongside equally high PPV and NPV values. These superior outcomes may be attributed to the smaller sample size, combined morphologic and diffusion assessment, and likely inclusion of distinct tumor subtypes, which can affect diffusion behavior and classification thresholds.